Your Location:Home > Products > Other > Potassium dimethyldithiocarbamate
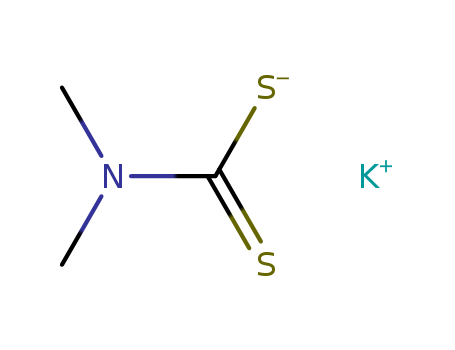


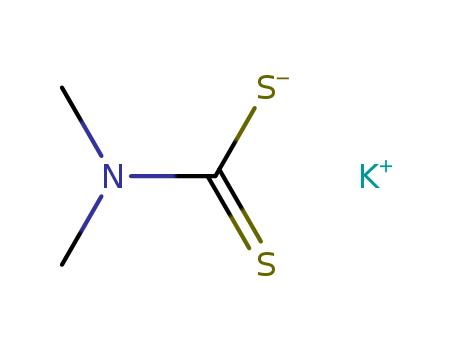
CasNo: 128-03-0
MF: C3H6KNS2
Appearance: clear light amber liquid
|
Air & Water Reactions |
Decomposes slowly to generate toxic hydrogen sulfide and dimethylamine. |
|
Reactivity Profile |
Potassium dimethyldithiocarbamate solution decomposes slowly to give hydrogen sulfide and dimethylamine. Decomposition is accelerated by acidification. Can generate flammable gases with aldehydes, nitrides, and hydrides. Incompatible with acids, peroxides, and acid halides. |
|
General Description |
A concentrated aqueous solution. A green liquid. |
InChI:InChI=1/C3H7NS2.K/c1-4(2)3(5)6;/h1-2H3,(H,5,6);/q;+1/p-1/rC3H6KNS2/c1-5(2)3(6)7-4/h1-2H3
-
Monoglyceride lipase (MGL) inhibition ma...
Reaction of 3-chloroacetamido-2-methyl-4...
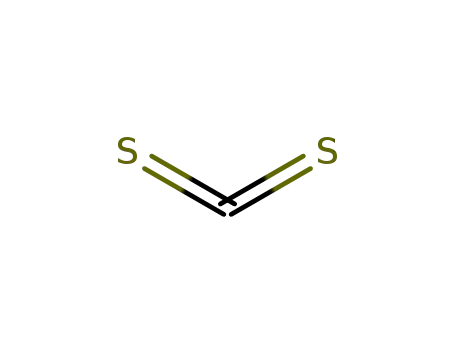
carbon disulfide

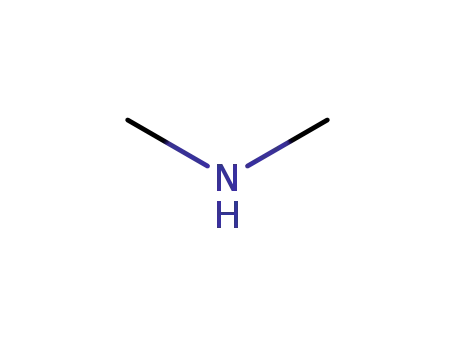
dimethyl amine

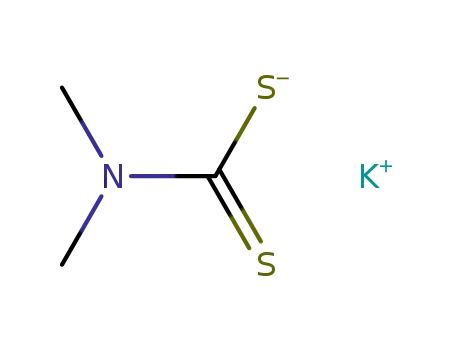
potassium dimethyldithiocarbamate
| Conditions | Yield |
|---|---|
|
With potassium hydroxide; In ethanol; for 1h; Ambient temperature;
|
|
|
With potassium hydroxide; In water; at 50 ℃; for 6h;
|
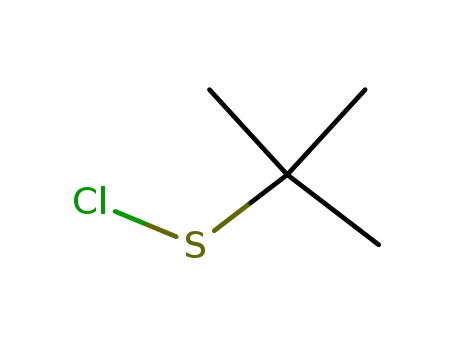
1,1-dimethylethanesulfenyl chloride


potassium dimethyldithiocarbamate

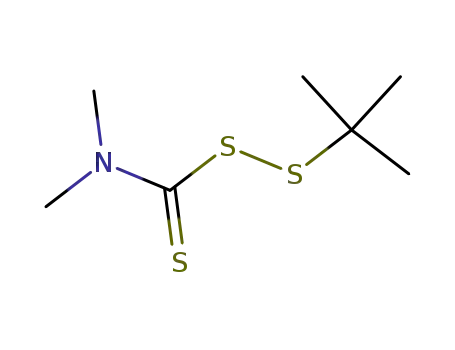
N,N-di-methyl-tert-butylsulfenyl dithiocarbamate
| Conditions | Yield |
|---|---|
|
In water;
|

carbon disulfide

dimethyl amine
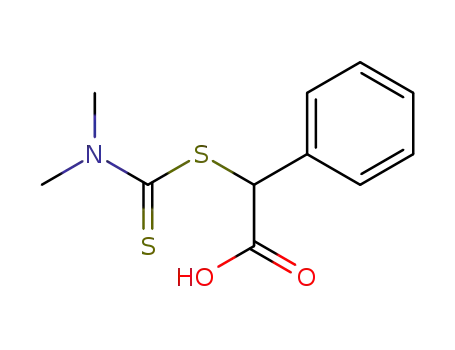
dimethylthiocarbamoylsulfanyl-phenyl-acetic acid
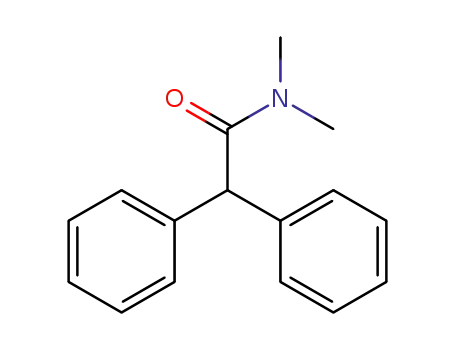
diphenamid

N,N-di-methyl-tert-butylsulfenyl dithiocarbamate
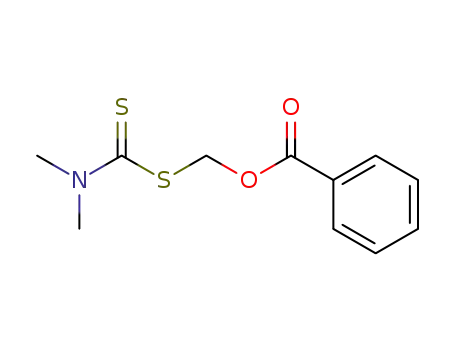
Dithiokohlensaeure-S-benzoyloxymethylester-dimethylamid